The importance of parts cleaning
In many manufacturing sectors, parts cleaning is critical to quality reassurance. Insufficiently cleaned parts can affect the quality of many subsequent industrial processes such as coating, welding, bonding and assembling. This can have a detrimental effect on the functionalities of the parts.
Particularly in highest-value manufacturing sectors such as aviation and defence, medical technology, and motor vehicles, cleaning quality is not only synonymous with quality, but reliability and even safety.
Which is better: Solvent or aqueous?
When deciding for the right cleaning method, many people instinctively ask: “Which is better – solvent or aqueous?”. The answer is: it depends. In fact, the question has never been about which one is better. It is rather: what are your requirements, goals and expectations and how can these demands be best fulfilled?
When it comes to parts cleaning, there is no one-size-fits-all solution. Remember you are not merely trying to do critical cleaning. You want to do the job in the most economically viable and safest way while meeting regulatory requirements and protecting the environment.
The right cleaning solution should balance technical, economic and safety requirements
So how should you go about selecting the right cleaning medium? We have developed a free guide outlining the 10 key questions you should ask during your decision-making process, addressing the technical, economic, as well as health and safety aspects. A brief look at some of the content includes:
1. What are your cleaning quality requirements?
In order to achieve optimal cleaning results, you need to consider two different kinds of soils:
1) particle contaminations, including their size, numbers and types and 2) the sort of filmy contaminations.
Aqueous cleaning agents have a density of approx. 1g/ml. Solvents, in comparison, may have a higher or lower density. “Heavier” cleaning agents are effective in removing particulates that cannot be readily dissolved. They can literally “push” or ”float away” particulates and lift them off the surface.
At the same time, different industrial applications require varying degrees of surface energy of the metal surface, which is influenced by filmy contaminations.
With nitriding, for example, a higher surface energy is required than with standard coating or assembling. The required surface energy should therefore match the ability of the cleaning agent. For precision cleaning where required surface energy can range anywhere from 38 mN/m to approx. 60 mN/m, both aqueous-based cleaning and solvents are capable of fulfilling the requirement.
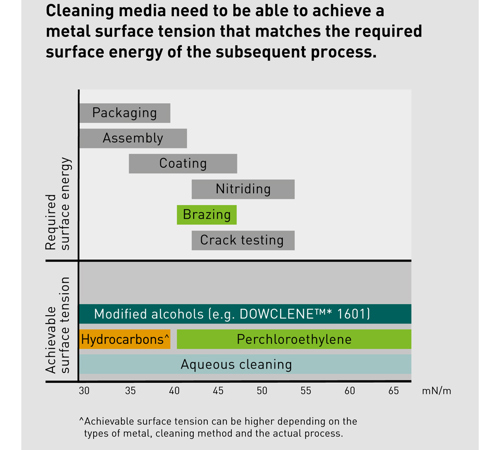
In aqueous cleaning, as soils and contaminations are emulsified and flooded off the surface which remain in the water (unless specific procedures are performed to purify the water), the quality of cleaning will mainly depend on the quality of the rinsing baths, as well as the number of the rinsing baths with demineralized water. The higher the required quality of cleaning, the investment and space requirements for the aqueous systems will increase accordingly.
2. What is the affinity of the cleaning agent to the soils?
Effective cleaning is based on the principle “Equal dissolves equal”. For water-based types of contaminations such as coolant and lubricant emulsions, aqueous cleaning agents are typically the first choice.
When removing mineral oil based, non-polar contaminations, such as machining oils, greases and waxes, solvent will commonly be the preferred cleaning agent.
Nowadays modified alcohols are also available; due to their both non-polar and polar properties, they are able to clean non-polar contaminations as well as certain polar contaminations. This type of solvents has a much broader application range compared to traditional non-polar solvents.

3. What metal types are you cleaning and how are they configured?
In water-based processes, cleaning agents which can be acidic, neutral or alkaline, are usually matched to specific metal types. Simultaneous cleaning of different metals can therefore be problematic and this can result in compatibility issues and in worst case: corrosion. Sometimes reactive additives are added to water to modify metal surface in processes such as edging and pickling. However, at times such reactive additives can also act aggressively on certain metal surfaces where it is not intended.
Solvents generally have broad material compatibility, which means they are compatible with all kinds of metal, when used properly, making it a suitable option for universal cleaning. If the component parts are tiny or have complex geometry or small crevices, solvent is often recommended due to its lower surface tension and viscosity which makes it easy to wet into and evaporate out of tight spaces.
With water-based cleaning, even tiny traces of residue moisture could give rise to issues such as challenges in subsequent production processes, corrosion, or growth of bacteria and related bioburden issues.
The above is only a brief summary. Download our free full guidance paper “10 key questions to consider when selecting the right cleaning agent” to help yourself establisha metal cleaning process that does not only provide consistent results, but is also economical and environmentally sustainable.











National Gas receives funding to develop Gravitricity underground hydrogen storage system
One single rock salt mine - Winsford - has 23 <i>MILLION </i>cubic metres of void and even allowing for 10% of that void set aside for hazardous waste...