A novel material that may demonstrate a highly unusual "liquid" magnetic state at extremely low temperatures has been discovered by a team of Japanese and US researchers.
The material, nickel gallium sulphide (NiGa2S4), was synthesized by scientists at
The scientists studied the polycrystalline sample using both X-rays and neutrons as probes to understand its structure and properties. The neutron experiments were conducted at the
The team found that the triangular arrangement of the material's atoms appears to prevent alignment of magnetic "spins," the characteristic of electrons that produces magnetism. A "liquid" magnetic state occurs when magnetic spins fluctuate in a disorderedly, fluid-like arrangement that does not produce an overall magnetic force. The state was first proposed as theoretically possible about 30 years ago. A liquid magnetic state may be related to the similarly fluid way that electrons flow without resistance in superconducting materials.
According to Collin Broholm, a professor in the Department of Physics and Astronomy at The Johns Hopkins University in
Each electron can be thought of as a tiny bar magnet. The direction of its "north" pole is its spin. "An ordered pattern of spins generally uses less energy, says Broholm, "but the triangular crystal structure prevents this from happening in this material."
The team conducted their neutron experiments with an instrument called a "disk chopper spectrometer." The only one of its kind in
A crystal diagram shows the triangle-shaped atomic structure of nickel gallium sulphide, which may have an unusual magnetic "liquid" state at low temperatures. Red spheres represent nickel, green spheres are gallium, and yellow are sulphur.
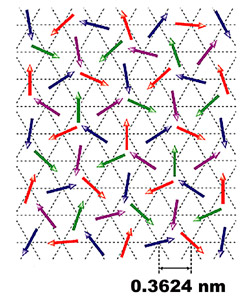
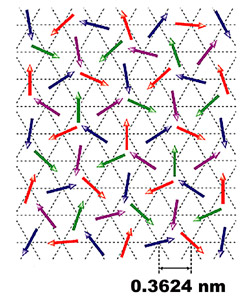
Multi-coloured arrows show the disordered array of magnetic spins of electrons within nickel gallium sulphide. The data were collected by precisely measuring the timing and change in direction of neutrons as they were passed through the material.




Nanogenerator consumes CO2 to generate electricity
Nice to see my my views being backed up by no less a figure than Sabine Hossenfelder https://youtu.be/QoJzs4fA4fo